ResMed Ltd. Recalls Continuous Positive Airway Pressure (CPAP) Masks
with Magnets due to Possible Magnetic Interference with Certain Medical Devices
The US Food and Drug Administration (FDA) has tagged ResMed’s recall of its magnet-containing Continuous Positive Airway Pressure (CPAP) masks as a Class I recall. Class I recalls are designated as having the potential for serious injuries or death if devices continue to be used. In November 2023, ResMed issued a field safety notice after discovering that the magnets in the masks could interfere with medical implants. In December 2023, the US-headquartered company went further and conducted a field correction. The recall, which in this case is a correction as opposed to a product removal, affects over 20 million of ResMed’s AirFit and AirTouch masks in the US, according to a 12 January FDA notice.
There have been six reported injuries and no deaths, according to the FDA.
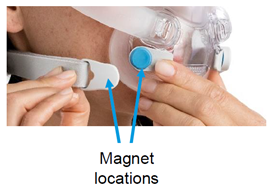
The masks use magnets to provide easier attachment and detachment to headgear. The company says this use is especially important to patients with dexterity vision impairment, or those with disabilities. The recall arose due to reports of magnetic interference of the magnets with implanted devices in the patient. ResMed said that the medical device function could be affected, whilst ferromagnetic implants could change position because of mask magnet proximity.
Active medical implants that could be affected include cardiovascular devices such as pacemakers, and implantable cardioverter defibrillators (ICD). Neurostimulators and cerebrospinal fluid (CSF) shunts can also suffer from interference, along with diabetes devices such as insulin/infusion pumps.
The FDA said that ResMed is recalling the devices to update labels, add additional warnings, and provide information to those who use the masks. This includes rules to keep the magnets at least six inches from implants or devices that could be affected.
Products Affected:
- Product Names: AirFit and AirTouch masks
- Product Codes: See Recall Database Entries:
- Class 1 Device Recall AirFit N10 (fda.gov)
- Class 1 Device Recall AirFit F20 (fda.gov)
- Class 1 Device Recall AirTouch F20 (fda.gov)
- Class 1 Device Recall AirFit N20 (fda.gov)
- Class 1 Device Recall AirTouch N20 (fda.gov)
- Class 1 Device Recall AirFit F30 (fda.gov)
- Class 1 Device Recall AirFit F30i (fda.gov)
- Model Numbers: AirFit N10, AirFit F20, AirTouch F20, AirFit N20, AirTouch N20, AirFit F30, AirFit F30i
- Distribution Dates: January 2020 to November 20, 2023
- Devices Recalled in the U.S.: 20,414,357
- Date Initiated by Firm: November 20, 2023
Device Use:
The AirFit and AirTouch masks are non-continuous ventilatory devices, intended to be used by patients weighing more than 66 lbs. who have been prescribed non-invasive positive airway pressure (PAP) therapy such as CPAP or bi-level therapy. The masks are meant for reuse by one person at home or by multiple people in hospitals. The devices have magnets on the lower headgear straps and frame connections of CPAP masks. These magnets are there to make wearing the mask more comfortable.
Reason for Recall :
ResMed Ltd. is recalling all their Continuous Positive Airway Pressure (CPAP) masks with magnets due to possible magnetic interference with certain medical devices. Under certain circumstances when a magnet is in close proximity (less than 2 inches) to certain medical implants and devices, it might disrupt their function or position, possibly causing serious harm or death.
While the existing label advises keeping magnets 2 inches away from affected medical devices, it doesn’t list all the specific ones that could be affected by the masks’ magnets. ResMed is recalling these masks to update the labels, add more warnings and information to guide patients and health care professionals on safe usage when using masks with magnets. This recall and label update came after a review of potential risks to medical implants due to magnetic interference.
The use of affected masks may cause serious adverse health consequences and death.
There have been six reported injuries. There have been no reports of death.
Who May be Affected:
- People who have been prescribed an AirFit or AirTouch mask for non-invasive positive airway pressure (PAP) therapy or bi-level therapy.
- Healthcare providers working in home-care or hospital settings who use an AirFit or AirTouch mask to administer non-invasive positive airway pressure (PAP) therapy or bi-level therapy.
What to Do:
On November 20, 2023, ResMed sent all affected customers an Important Medical Device Advisory.
The letter requested customers to:
- Keep the magnets at a safe distance of six inches (150 mm) away from implants or medical devices that could be affected by magnetic interference.
- Patients should reach out to their physician or the manufacturer of their implant or other medical device for further details regarding possible negative effects of magnetic fields on their device.
- Visit the ResMed Magnet Update website for detailed information about the recent updates made to contraindications and warning labels.
Contact Information:
Customers in the U.S. with questions about this recall should contact ResMed at call 1-800-424-0737.